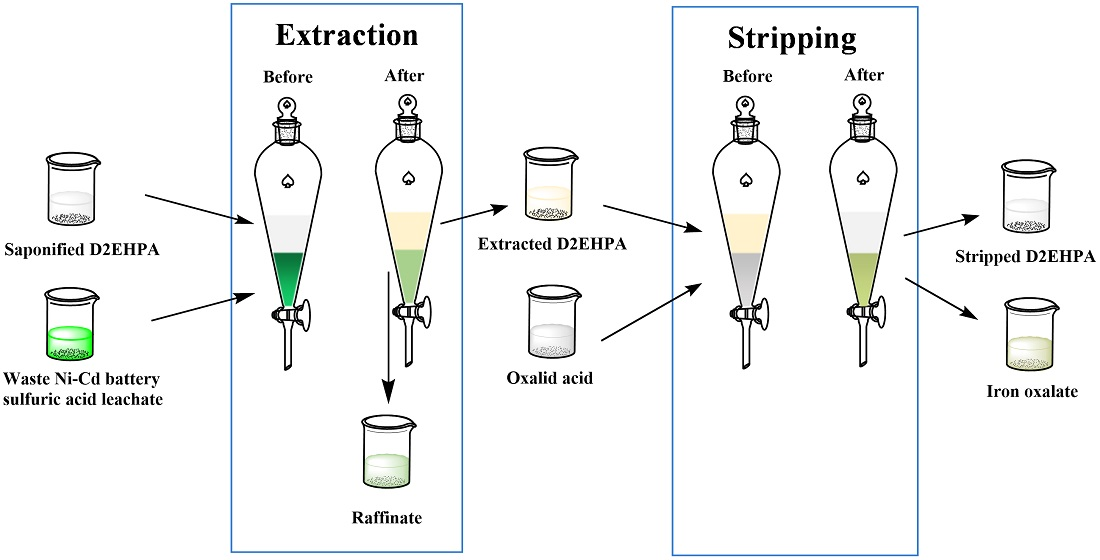
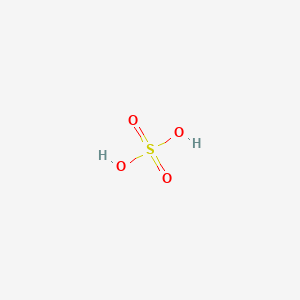
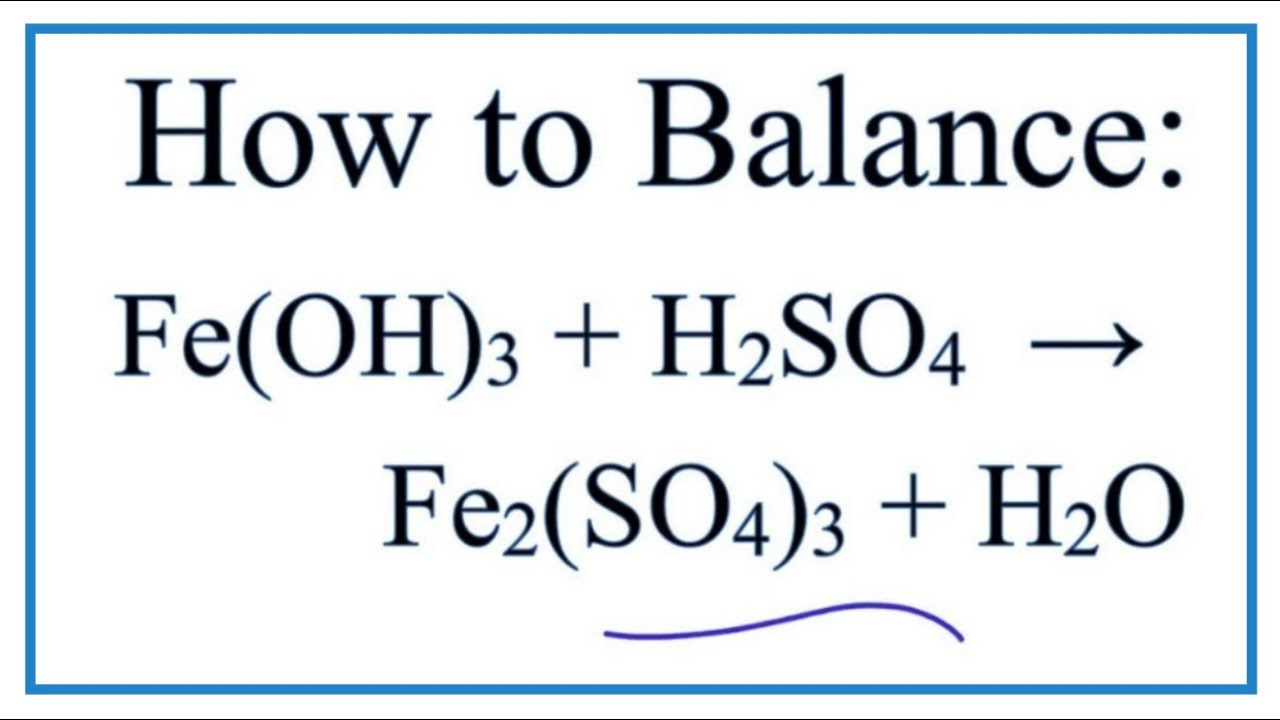
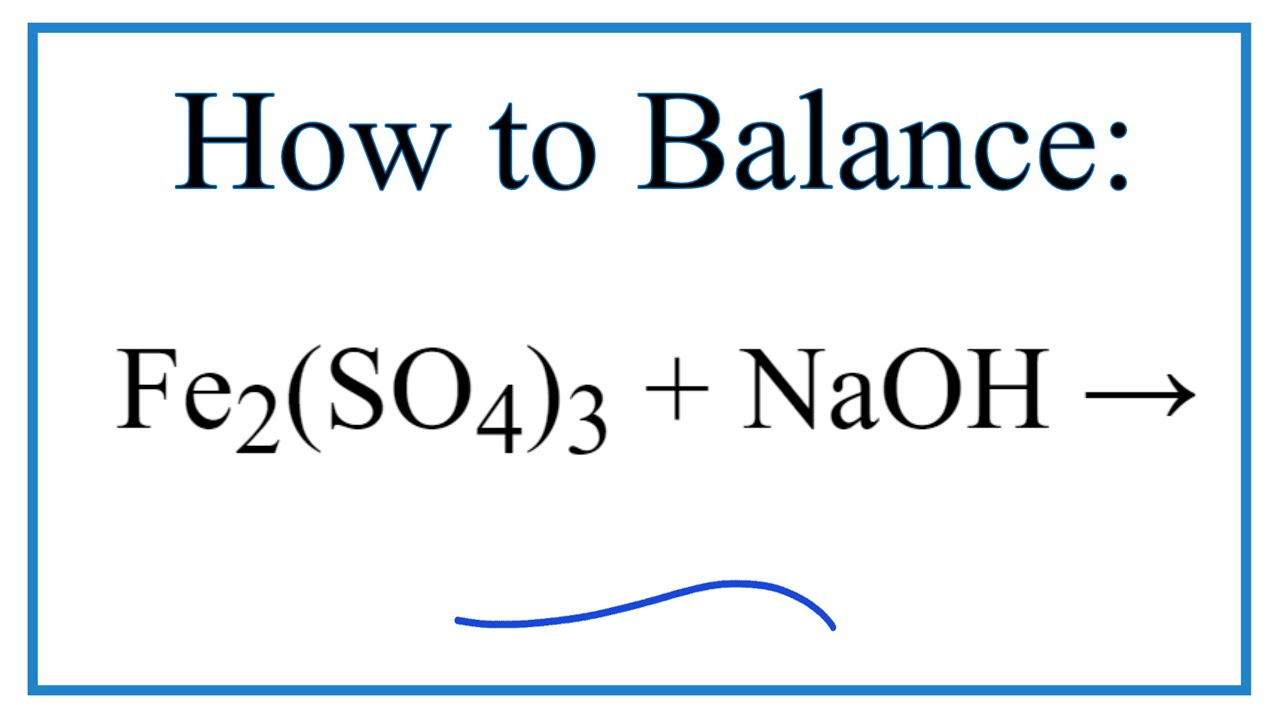
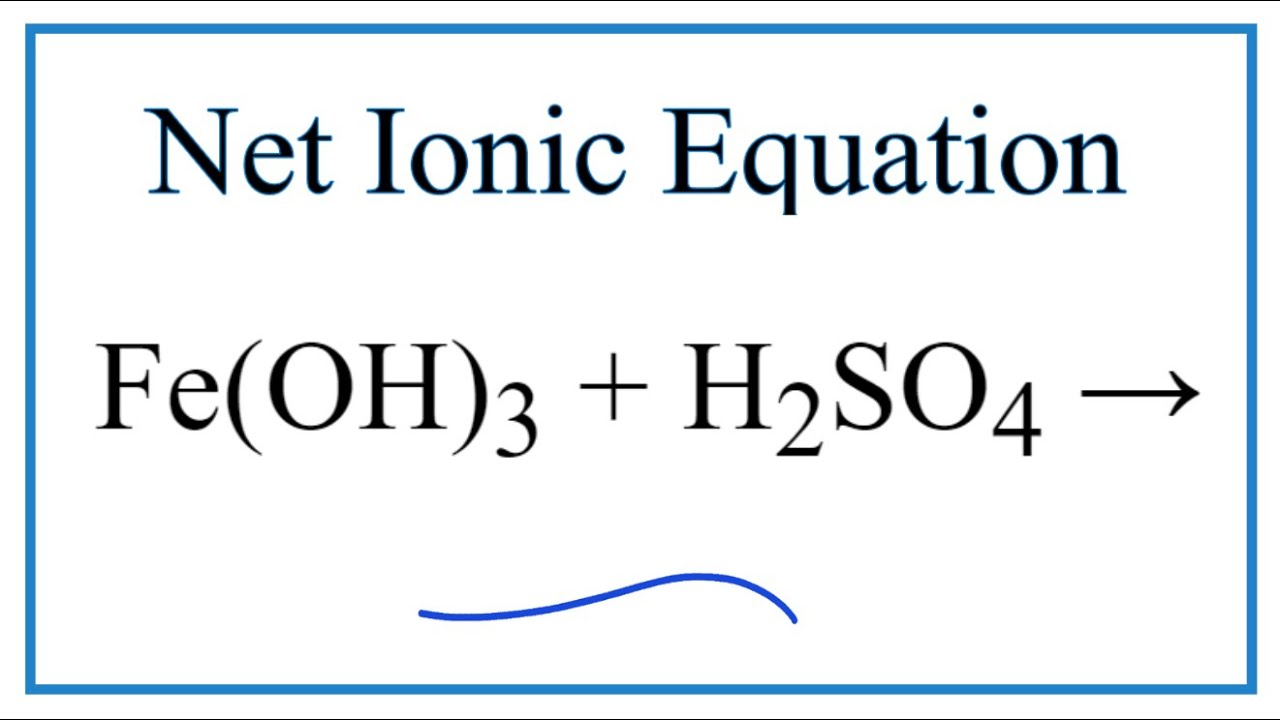Fe2O3+H2SO4=Fe2(SO4)3+H2O Balanced Equation|| Balanced equation for Iron iii oxide and Sulfuric acid - YouTube
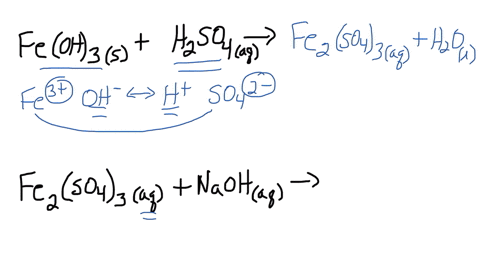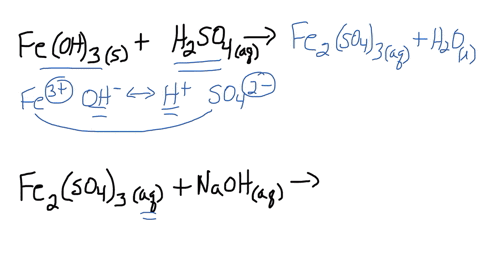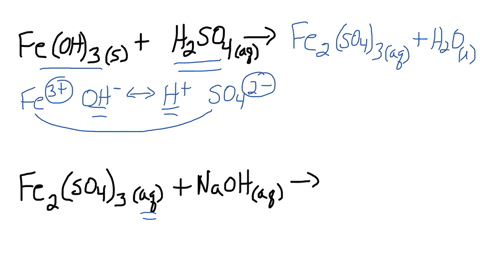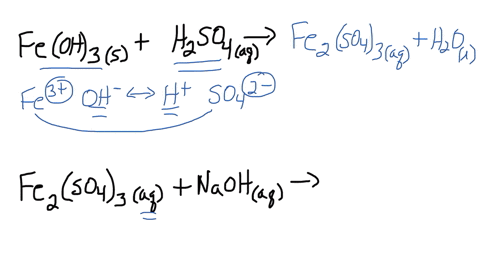
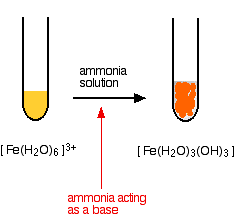
SOLVED: Iron(III) sulfate is made in industry by the neutralization reaction between solid iron(III) hydroxide and aqueous sulfuric acid. The iron(III) sulfate is then added with sodium hydroxide to municipal water in

The process of pyrite weathering in a deep metal mine. Four general... | Download Scientific Diagram

Effect of sulfuric acid concentration on iron dissolution and final pH.... | Download Scientific Diagram