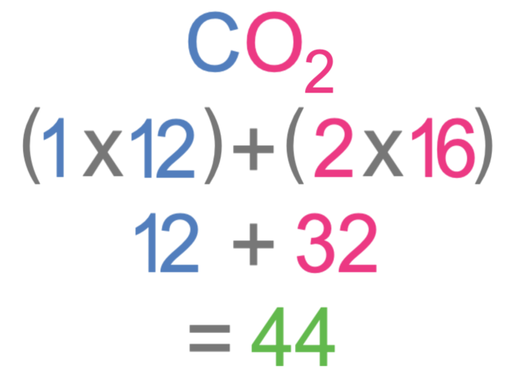Take your periodic table out. What is atomic mass of Carbon Point where you can find it in the periodic table! 6 is atomic number not atomic mass Atomic. - ppt download
An organic compound contains element C, H and oxygen. A 4.24 mg sample of compound is completely burnt in oxygen. It gives 8.45 mg of carbon dioxide and 3.46 mg of water.

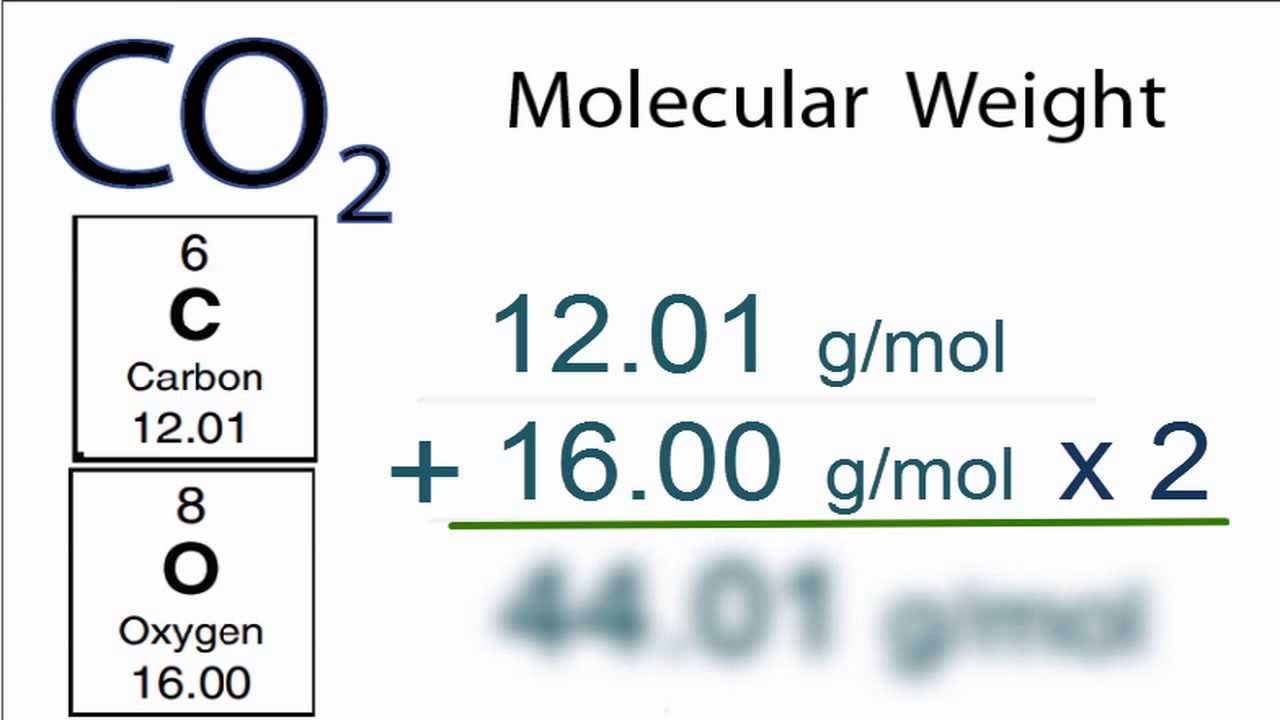
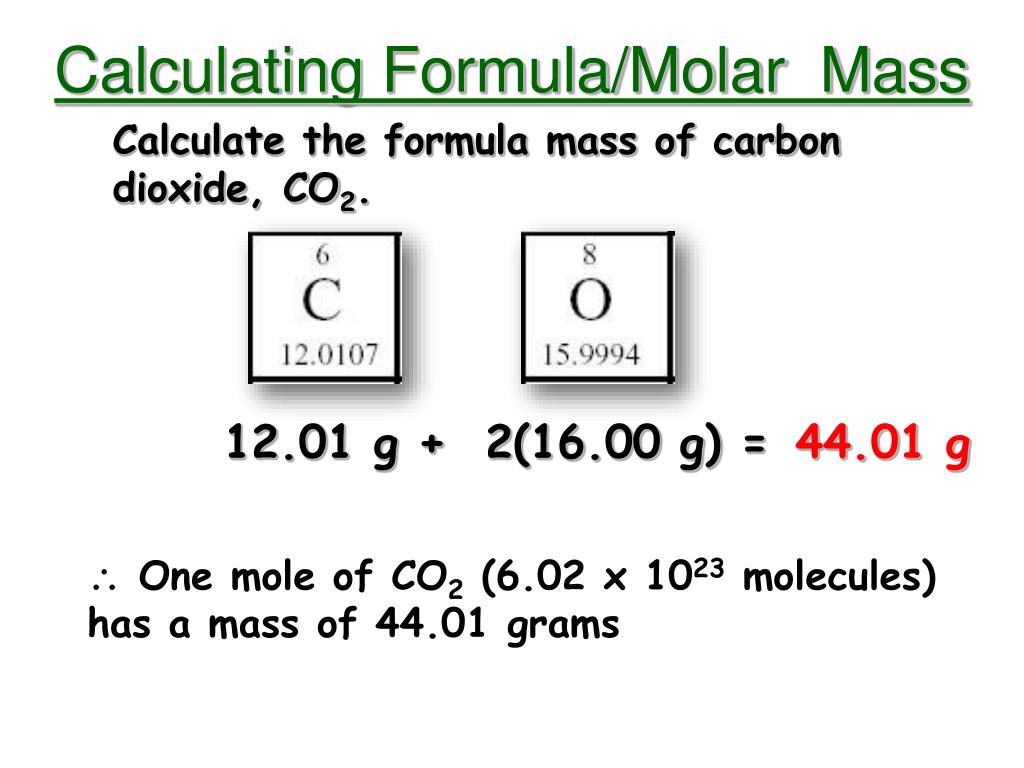
The Mole Calculating Formula/Molar Mass Calculate the molar mass of carbon dioxide, CO g + 2(16.00 g) = g One mole of CO 2 (6.02 x ppt download





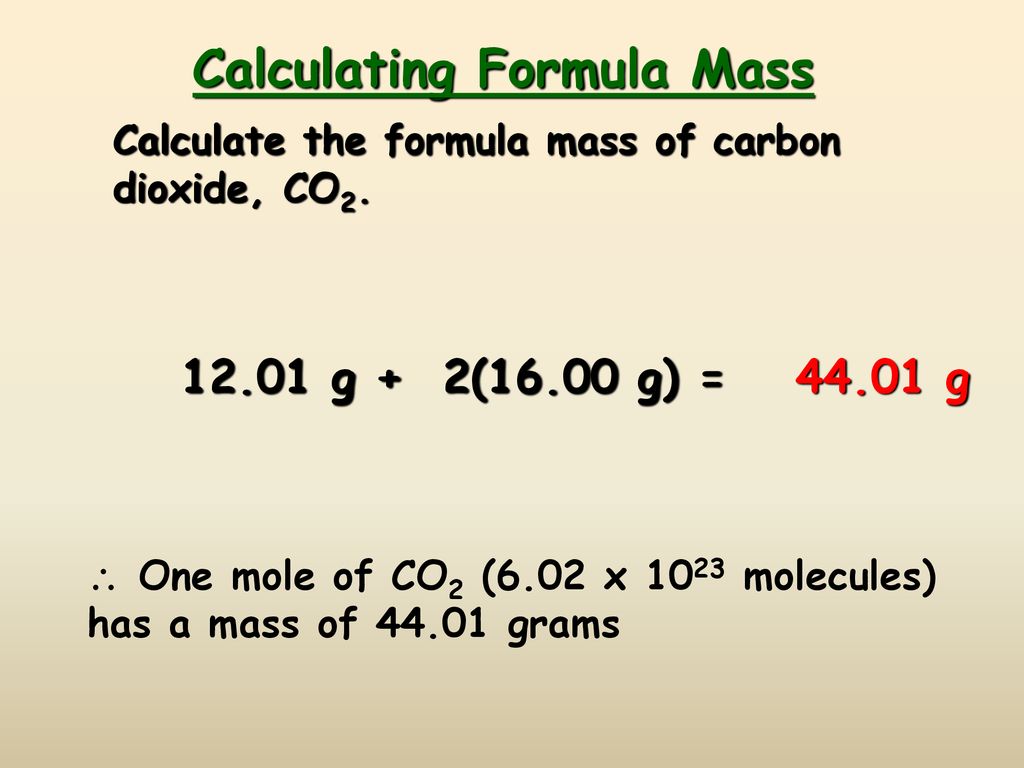





![Carbon Monoxide [CO] Molecular Weight Calculation - Laboratory Notes Carbon Monoxide [CO] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2021/11/carbon-dioxide-molecular-weight-calculation.jpg)