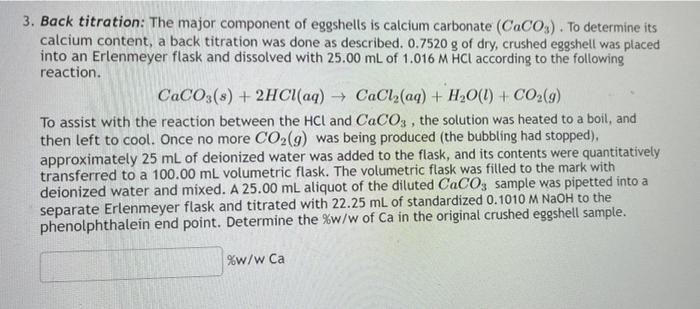
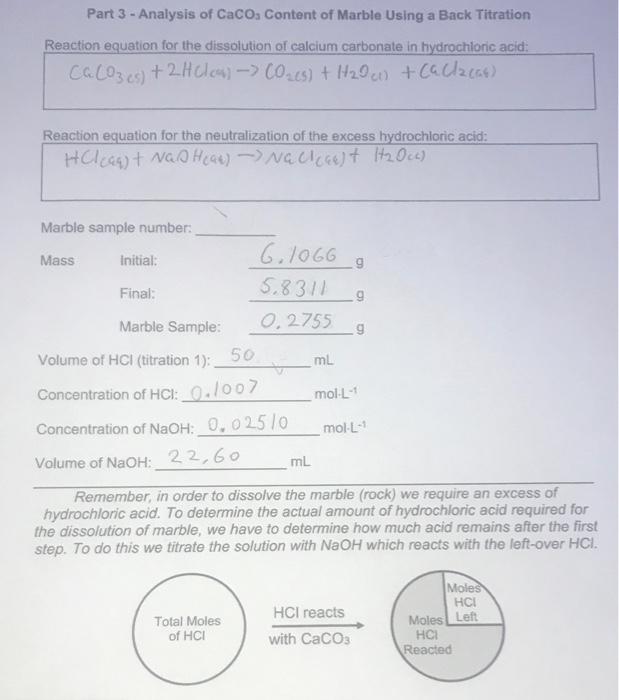
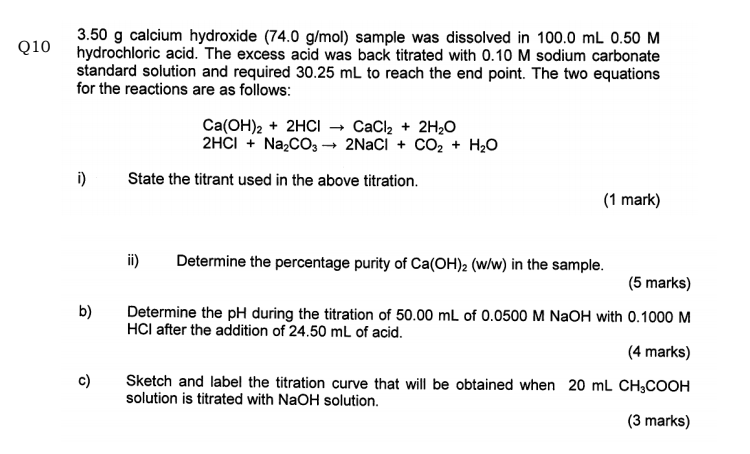
Experiment Report - back titration.docx - Experiment Report Finding the Purity of CaCO3 using Back Titration Introduction In order to calculate the | Course Hero

DOC) OBJECTIVE To determine the calcium carbonate content in eggshell | Sharifah Hana - Academia.edu

The relationship between the log amount of pure calcium carbonate and... | Download Scientific Diagram

Back-Titration F22 - Copy - Back-Titration of a Commercial Antacid Stephanie R. Geggier New York - Studocu

Determination of Amount of CaCO3 in Eggshell by Back Titration Method | PDF | Hydrochloric Acid | Titration

An Extensive Indirect Titration Report Exemplar Regarding the Mass of Calcium Carbonate in Antacids, Received a Final Grading of A+. | Chemistry - Year 12 SACE | Thinkswap
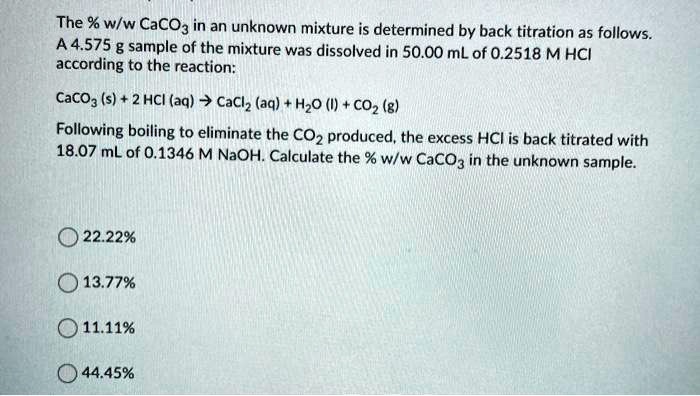
SOLVED: The % w/w CaCO3 in an unknown mixture is determined by back titration as follows: A 4.575 g sample of the mixture was dissolved in 50.00 mL of 0.2518 M HCl