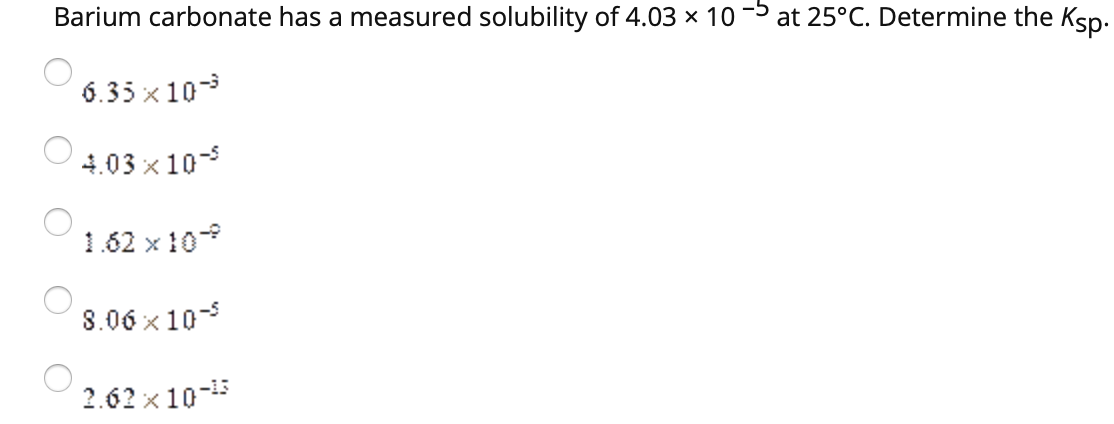Solubility in molten lithium carbonate of various oxides and calcium... | Download Scientific Diagram

Thermogravimetric curves for sample, residues of fractionation based on... | Download Scientific Diagram

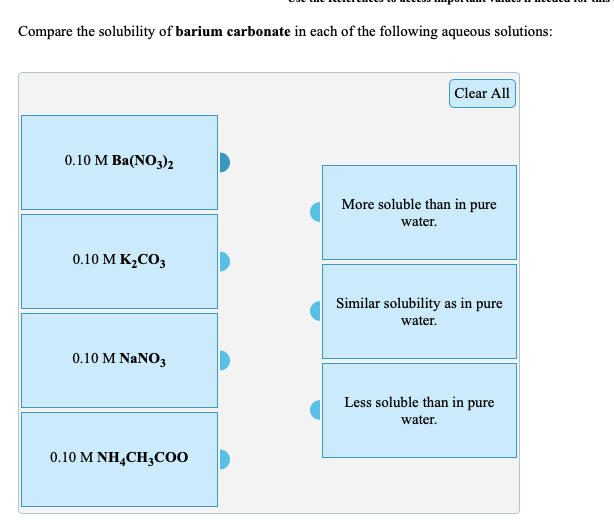
Section 4: Solubility Equilibrium. Objectives Explain what is meant by solubility product constants, and calculate their values. Calculate solubilities. - ppt download

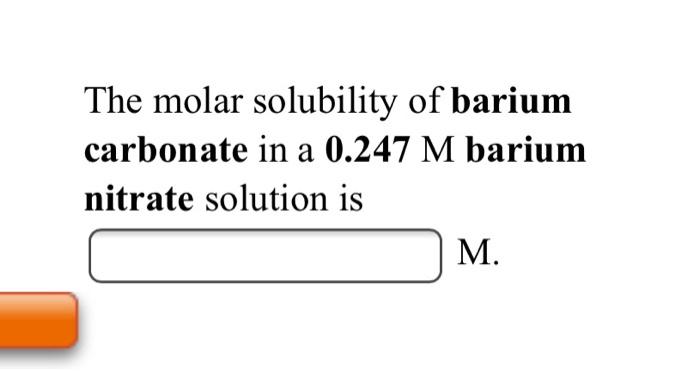
OneClass: Calculate the solubility of barium carbonate, BaCO3 in units of grams per liter. Ksp(BaCO3)...
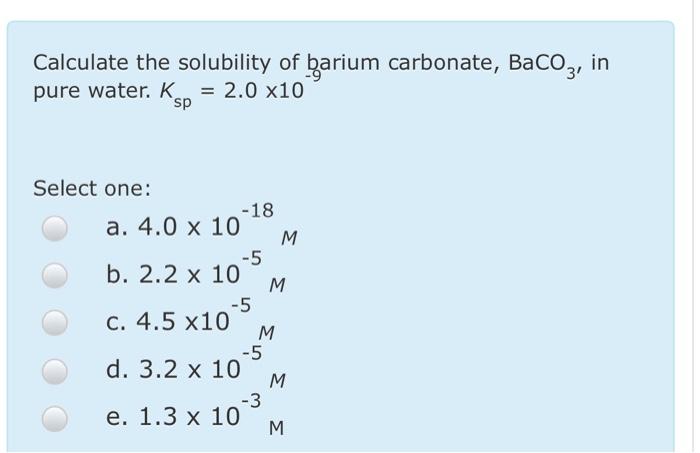
SOLVED: Calculate the solubility of barium carbonate, BaCO3, in pure water. Ksp = 2.0 × 10^-9 a. 2.2 × 10^-5 M b. 3.2 × 10^-5 M c. 1.3 × 10^-3 M d. 4.0 × 10^-18 M e. 4.5 × 10^-5 M

Solubility of common oil field scales of injection water and high-barium concentration and high-salinity formation water | Semantic Scholar